Let us answer your questions!
QReview can reduce business and compliance risk by improving the quality and clarity of your documentation.
Our partners in the medical device, pharmaceutical and biologics industries will from on-demand remote access to high-quality resources without the costs of consultant travel and processing new contracts and POs for each review or consulting event. Benefits include:
- On-demand access to QualityHub’s expansive network of subject matter experts experienced in performing critical reviews.
- Reduction in costs associated with maintaining consultants in travel status.
- Improved inspection and audit readiness of important records and documents.
- Mitigation of workflow bottlenecks and backlogs.
- Opportunity for an unbiased quality review (i.e. independence from the current working team).
- Improved documentation and outputs to meet regulatory guidelines, expectations, and best industry practices.
- Independent assessment of critical documentation that drives decision making.
- Improve clarity and content of documentation and records.
- Increased confidence that the right objective evidence is provided to support actions taken.
- Reduction in document rework or reject by approves.
- Reduction to the client’s carbon footprint.
We can provide a variety of remote auditing & consulting resources depending on the type of review or consult submitted. QualityHub has an excellent reputation for technical and regulatory knowledge and ability to interpret regulatory requirements and apply these to a variety of product types.
We have experts in most of the life sciences areas, as well as former FDA staff, RA/QA professionals, healthcare professionals, biologists, chemists and engineers. Our experts have broad experience in product development, production, testing, engineering, quality, purchasing, training, operations and also pre and post-market requirements for medical devices, pharmaceuticals and biologicals. We will be “at your service” remotely on an “as-needed” basis.
You will receive the consultant’s resume (qualifications) for your records and if necessary you can request an alternate resource. In most cases, we will keep the same reviewer working with you to ensure continuity as long as the document types or technical subject matter remains the same.
Our reviewers have been trained on our unique QReview process and are ready to help.
- We will work with you to quickly get an NDA and Subscription Service Agreement in place.
- Upon approval, QualityHub provides access to a client-specific SharePoint folder (or we can accommodate the use of your companies file management system or limit information to transfer by email).
- On an as-needed basis, your team members can submit a QReview Review/Consult Request Forto specify the types and number of documents you would like reviewed, or the topic you wish to discuss with one of our consulting staff. Our project management team will quickly process your request.
- For significant “business-critical” documents you can also request a peer review. If requested, an expert or an executive level consultant will provide a secondary review of the consultants’ deliverables to add commentary, and, if needed, verify alignment of content with the appropriate regulations. (This might be helpful for documents that are particularly business-critical.)
- We match your request to the appropriate SME(s) and upload their resume(s) for your files.
- Documents are uploaded to a secure location, and, as-needed, conference or video calls are set-up for consultation or desk-top sharing. The SME provides the requested review or assistance, returning redlines or comments.
- Follow up meetings can be set up, as needed. Turn-around times for document reviews are intended to be short but depend on document complexity.
- QualityHub monitors the utilization of hours against your subscription package, providing periodic balance or expiration updates.
QualityHub understands the importance of client confidentiality and the protection of proprietary information.
QualityHub’s strict guidelines and processes ensure confidentiality is maintained during and after the document review and/or consultation process:
- QReview provides a secure, encrypted means for transferring and storing documents and records on a secure cloud-based platform.
- When processing your subscription, QualityHub will establish a client-specific confidentiality and non-disclosure agreement. This can be done using your NDA or ours.
- All of our SMEs and consultants have signed confidentiality and nondisclosure agreements that extend from QualityHub to all of our clients, projects and associated information.
- The subscription service agreement includes reference to the protection of confidential and proprietary documentation.
We know flexibility is important. It can be challenging to predict how much consulting time you will need in a year.
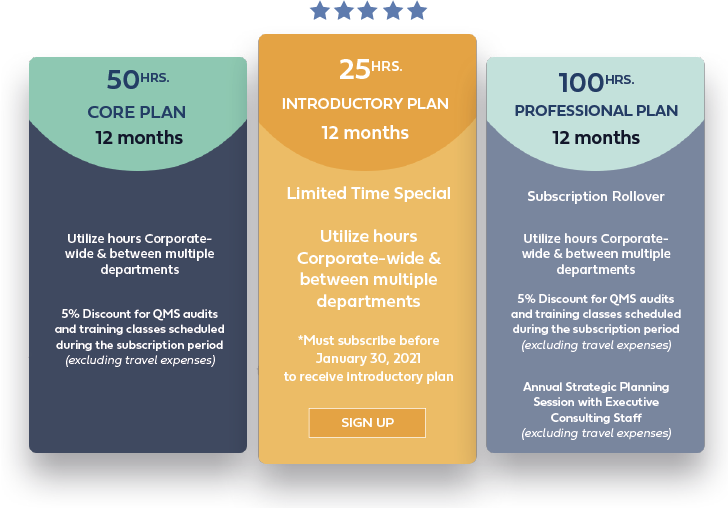
We have two subscription packages that allow you to choose either 50 or 100 hours of review/consult service.
We suggest choosing the plan that fits your needs, and we are happy to help guide you through that process. Once the subscription has begun, it will be up to you to make requests for our services as time progresses during the plan.
Per our policy, you can choose to use or to not to use the remaining hours on your subscription. We will do our best to notify you of hours that are remaining throughout the subscription period.
Our goal is always to meet or exceed our customers’ expectations. We will do our best to resolve any concerns you might have regarding your subscription service.
You may cancel your subscription at any time. However, per our policy if you cancel your subscription, we do not provide refunds for unused hours.
If you are unable to use your hours within the subscription time period, you may choose to renew for additional calendar year and apply those unused hours to your subscription time period. Please see the Roll-Over section for more information.